الهرمونات الذكرية لدى النساء

- Male hormones are referred to as androgens.
- The most well-known male hormones are testosterone and androstenedione.
- These male hormones are present in women as well as men, and they are essential for maintaining the right hormonal balance.
- Symptoms of excess male hormones include acne, alopecia (hairloss) and hirsutism (excess hair).
- 70% of women with PCOS experience excess male hormones (hyperandrogenism) and 10-30% of cases of excess male hormones will be due to a different disorder.
What do male hormones (androgens) do in women?
In women, androgens are secreted by the ovaries and the adrenal gland. Testosterone is a precursor of oestradiol and therefore, the availability of testosterone has a direct effect on how much oestradiol is produced. Male hormones are involved in enhancing a woman’s libido and maintaining a healthy reproductive system. They are implicated in puberty and, given intra vaginally, they can improve signs of vaginal atrophy during the menopause.
Low androgen levels in women
The importance of androgens in women is demonstrated by the ill effects that are felt when their synthesis is disrupted in any way. An androgen deficiency in females can cause a reduction in sexual desire (low libido), fatigue and a general lowering of mood. There are limited, small scale studies suggesting that androgens exert protective effects on the heart and brain; and low testosterone concentrations have also been associated with a decline in bone mineral density. These studies all require further validation, but they do hint at a physiologically beneficial role for testosterone and the other androgens in females.
Serum levels of androstenedione drop markedly after the menopause, however, levels of testosterone are usually maintained, which suggests that the ovaries are still producing testosterone. Women who undergo an oophorectomy typically experience a fall in serum testosterone levels of approximately 50%, as production shifts entirely to the adrenal gland.
Whilst the prospect of androgen replacement therapy seems appealing, to date, it is only recommended for the treatment of hypoactive sexual desire disorder and not for other cases of suspected androgen deficiency.
High androgen levels in women
The term applied to those with an excess of androgens is hyperandrogenism. The clinical manifestations of this can include acne, hirsutism and alopecia. Biochemically, the condition is defined by an increase in circulating androgens in the serum.
Excess androgens can have a long-term effect on health, increasing the risk of conditions including type 2 diabetes, high blood pressure and heart disease (somewhat confusingly, both abnormally low and abnormally high testosterone levels have been linked to an increased risk of cardiovascular disease). The impact of high androgen levels on a female’s self-confidence should also not be overlooked. Women who are experiencing significant alopecia or hirsutism are at increased risk of struggling with image-related insecurities.
There are a number of recognised causes of hyperandrogenism, these include:
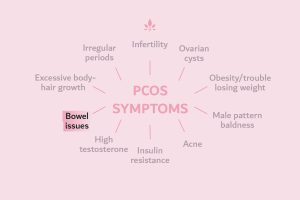
Androgens (male hormones) and Polycystic Ovary Syndrome (PCOS)
Perhaps the most well known disorder of androgen excess is PCOS. Approximately 70% of women with hyperandrogenism (excess male hormones) will receive a diagnosis of PCOS. The condition can be challenging to both diagnose and manage, as it is a syndrome, which presents differently from patient to patient. In fact, diagnosis is usually performed by a process of exclusion, whereby other possible conditions are systematically ruled out.
According to the Rotterdam criteria, to be diagnosed with PCOS, a female must present with two out of the following three symptoms:
- Hyperandrogenism (excess male hormones)
- Anovulation (lack of ovulation)
- Polycystic ovaries.
However, other guidelines, such as those by the National Institute of Health, consider the condition to be primarily one of androgen excess, which in turn gives rise to the other physical characteristics of the syndrome.
The preferred approach for the management of PCOS is making lifestyle adjustments, such as losing weight. This has been shown to significantly improve the symptoms of the condition.
Whilst the majority of women with hyperandrogenism will have PCOS, 10-30% of cases of androgen excess will be due to a different disorder.
Congenital Adrenal Hyperplasia
The Congenital Adrenal Hyperplasias (CAHs) are a group of disorders characterised by impaired cortisol secretion because the enzymes that are normally responsible for its production are missing or ineffective. These disorders are autosomal recessive, which means that to inherit them you need to inherit a mutated gene from both parents. Whilst the classical form most often presents in childhood, the non-classical form is usually diagnosed after puberty, or in adulthood, with symptoms very similar to those seen in women with PCOS, including hyperandrogenism, infertility and disrupted menstrual cycles. In CAH the adrenal gland is the source of the excess androgens, rather than the ovaries.
The prevalence varies according to ethnicity, with Ashkenazi Jews and Europeans of Latin descent at most risk. Between 1 and 10% of women with hyperandrogenism will discover that they have non-classical CAH.
Androgen-secreting neoplasms
Androgen-secreting neoplasms of the ovary or adrenal gland are rare. Ovarian androgen-secreting neoplasms are responsible for between 0.1 and 0.3% of cases of hyperandrogenism; adrenal androgen-secreting neoplasms are even rarer than this. It is, however, important to check for their presence as some are cancerous and will require urgent treatment.
The symptoms of neoplasms often mimic those of PCOS, but their onset is usually rapid and effects worsen with time. Women with these sorts of neoplasms will usually experience severe hirsutism, alopecia and acne. They may also observe a change in body shape, with breasts becoming smaller and a loss of feminine body contours.
Ovarian neoplasms might be palpable during a pelvic exam, otherwise they should be easy to identify using ultrasound.
Cushing’s Syndrome
Cushing’s syndrome is a rare condition characterised by an excess of cortisol. This leads to an increase in the secretion of adrenal androgens, contributing to symptoms such as hirsutism and acne that are synonymous with hyperandrogenism.
Fewer than 1% of women with hyperandrogenism will be diagnosed with Cushing’s syndrome.
Insulin Resistance
Approximately 3% of women with hyperandrogenism will be diagnosed with hyperandrogenic-insulin resistant-acanthosis nigricans syndrome. Women with this condition have severe metabolic abnormalities, are usually overweight and are at increased risk of developing type 2 diabetes mellitus (T2DM). They have extensive acanthosis nigricans and are usually severely hyperandrogenic, with some virilisation, meaning that they are developing male characteristics, such as a deep voice and increased muscle mass.
A condition of high insulin resistance will normally be diagnosed by measuring the levels of circulating insulin. The long term consequences of the condition, such as hypertension and T2DM, necessitate prompt and effective management. Women diagnosed with PCOS are prone to have insulin resistance and develop type 2 diabetes mellitus (T2DM).
Idiopathic hyperandrogenism
Some women exhibit symptoms of hyperandrogenism but do not fulfil the criteria for PCOS and do not experience other PCOS symptoms or the other conditions described above. In these cases, the reason for their androgen excess remains unknown and they are diagnosed with idiopathic hyperandrogenism.
Environmental androgens/endocrine disruptors
Endocrine disruptors are a cause of great concern, due to their widespread prevalence. They are found in a large number of everyday cleaning and beauty products and they exert their detrimental effects by upsetting the normal hormonal balance.
Many environmental androgens pose an additional risk because they also cross the placental barrier, creating a potentially harmful hyperandrogenic foetal environment. Long-term effects are unclear, but future metabolic and reproductive disorders are a concern for these babies.
Two well characterised examples of endocrine disruptors that have androgenic activity are triclocarban (TCC) and nicotine. TCC is an antimicrobial found in, amongst other products, soaps, clothing, carpets and plastics. It seems to regulate the activity of the androgen receptors, affecting the availability of testosterone. Nicotine is found in cigarettes and can cross the placental barrier, accumulating in the amniotic fluid. Women smokers have increased testosterone levels.
Nabta is reshaping women’s healthcare. We support women with their personal health journeys, from everyday wellbeing to the uniquely female experiences of fertility, pregnancy, and menopause.
Get in touch if you have any questions about this article or any aspect of women’s health. We’re here for you.
Sources:
- Azziz, Ricardo, et al. “The Androgen Excess and PCOS Society Criteria for the Polycystic Ovary Syndrome: the Complete Task Force Report.” Fertility and Sterility, vol. 91, no. 2, Feb. 2009, pp. 456–488., doi:10.1016/j.fertnstert.2008.06.035.
- Davis, Susan R, and Sarah Wahlin-Jacobsen. “Testosterone in Women—the Clinical Significance.” The Lancet Diabetes & Endocrinology, vol. 3, no. 12, 7 Sept. 2015, pp. 980–992., doi:10.1016/s2213-8587(15)00284-3.
- Galan, N. “Late-Onset Congenital Adrenal Hyperplasia .” Very Well Health, www.verywellhealth.com/congenital-adrenal-hyperplasia-overview-2616550. Updated December 20, 2018.
- Hammes, Stephen R., and Ellis R. Levin. “Impact of Estrogens in Males and Androgens in Females.” Journal of Clinical Investigation, vol. 129, no. 5, 1 May 2019, pp. 1818–1826., doi:10.1172/jci125755.
- Hewlett, M, et al. “Prenatal Exposure to Endocrine Disruptors: A Developmental Etiology for Polycystic Ovary Syndrome.” Reproductive Sciences, vol. 24, no. 1, Jan. 2017, pp. 19–27., doi:10.1177/1933719116654992.
- Snyder, Peter J. “Editorial: The Role of Androgens in Women.” The Journal of Clinical Endocrinology & Metabolism, vol. 86, no. 3, 1 Mar. 2001, pp. 1006–1007., doi:10.1210/jcem.86.3.7369.
- Witchel, S F. “Nonclassic Congenital Adrenal Hyperplasia.” Current Opinion in Endocrinology, Diabetes and Obesity, vol. 19, no. 3, June 2012, pp. 151–158., doi:10.1097/MED.0b013e3283534db2.